
Do More for Your
MGD Patients
Systane® iLux²® is the all-in-one, portable device that can help eye care professionals evaluate and treat MGD via customizable heat and expression of the meibomian glands.
![]()
8-12 MIN TREATMENT1,2
![]()
12-MONTH EFFICACY
![]()
IMAGING USED FOR
MEIBOGRAPHY
Meet Systane® iLux²®
Provide Fast and Lasting Treatment to Your MGD Patients
Look to the world’s first meibomian gland dysfunction (MGD) thermal pulsation system with imaging that can be used for meibography + gland treatment + HD video replay - all in the palm of your hand.
The Systane® iLux²® is a portable, hand held device designed to help eye care professionals evaluate and treat MGD. The meibomian gland imaging capabilities allow patients to see the condition of their glands, helping educate them on the need for treatment.
The Systane® iLux²® system allows you to target dysfunctional meibomian glands, improving signs and symptoms of MGD through customizable, localized heat and compression therapy. It delivers fast and efficacious treatment,1,2 while allowing patients to visualize their results post procedure through HD video replay.
Place the Systane® iLux²® in your space using Augmented Reality!
To access the experience, open ilux.myalcon.com/ecp/ on the desktop browser and scan the QR code using your mobile phone.

Key Features


All-in-one, portable device
Systane® iLux2® MGD Thermal Pulsation System gives you personalized treatment with HD video replay and gland imaging.
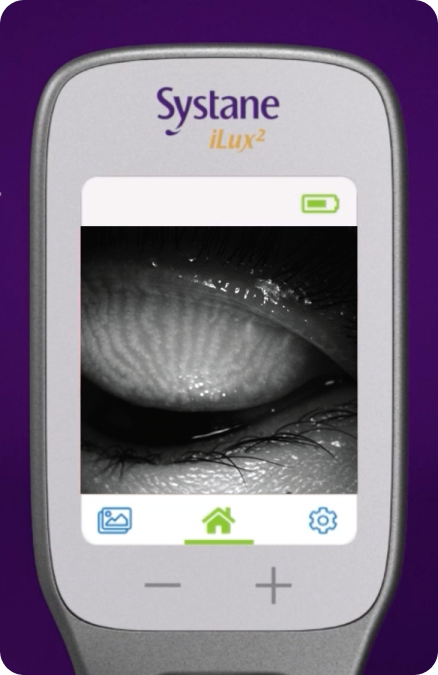

Patient education
Systane® iLux²® can image meibomian glands to help patients understand and see the results after the procedure.


Fast and efficacious
Treatment of both eyes using the Systane® iLux²® takes approximately 8-12 minutes.1,2 A single treatment can result in an increase in meibomian gland secretion, improvement in tear film stability, and reduction in dry eye symptoms as early as one week and can last for up to 12 months.3,4


You’re always
in control
The Systane® iLux²® device lets you customize heat and expression treatments across each easy-to-visualize treatment zone, allowing you to target therapy where the patient needs it most.
Patient Outcomes
Take control of dry eye treatment with Systane® iLux2® and see the results
Significant improvements in symptoms after one week. Post-treatment Meibomian Gland Score (MGS) increased on average by 315% versus baseline4*

Increased average meibomian gland function by 300% at 4 weeks post-treatment, compared to baseline5**

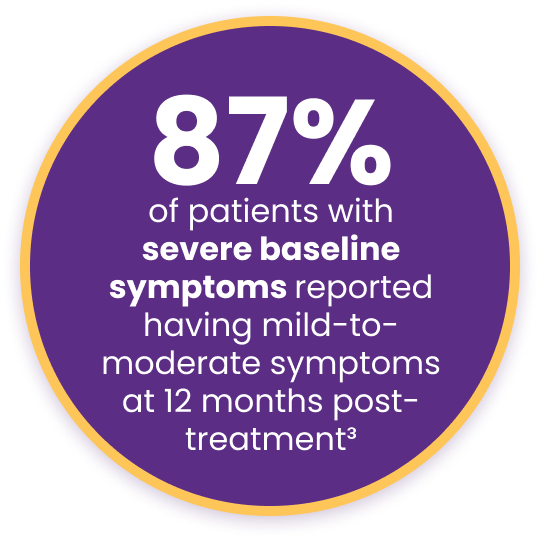
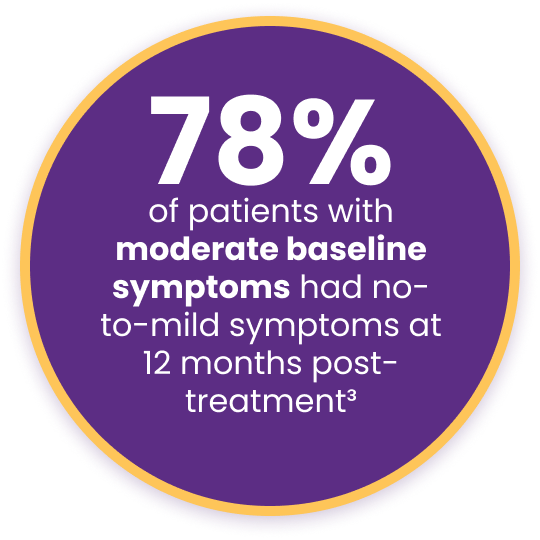
Improved meibomian gland secretion score (MGS) by more than 17 points at 12 months after treatment3†

*(P<0.0001). In a prospective, open-label, multicenter study of patients ≥18 years with MGD (n=30).
**Measured at baseline, 2 weeks, and 4 weeks.
†Meibomian gland scores (0-45) measured using Meibomian Gland Evaluator to assess the 15 glands of the lower lid of each eye with a grade from 0-3.
Who Might Benefit Most from Treatment with Systane® iLux²®?
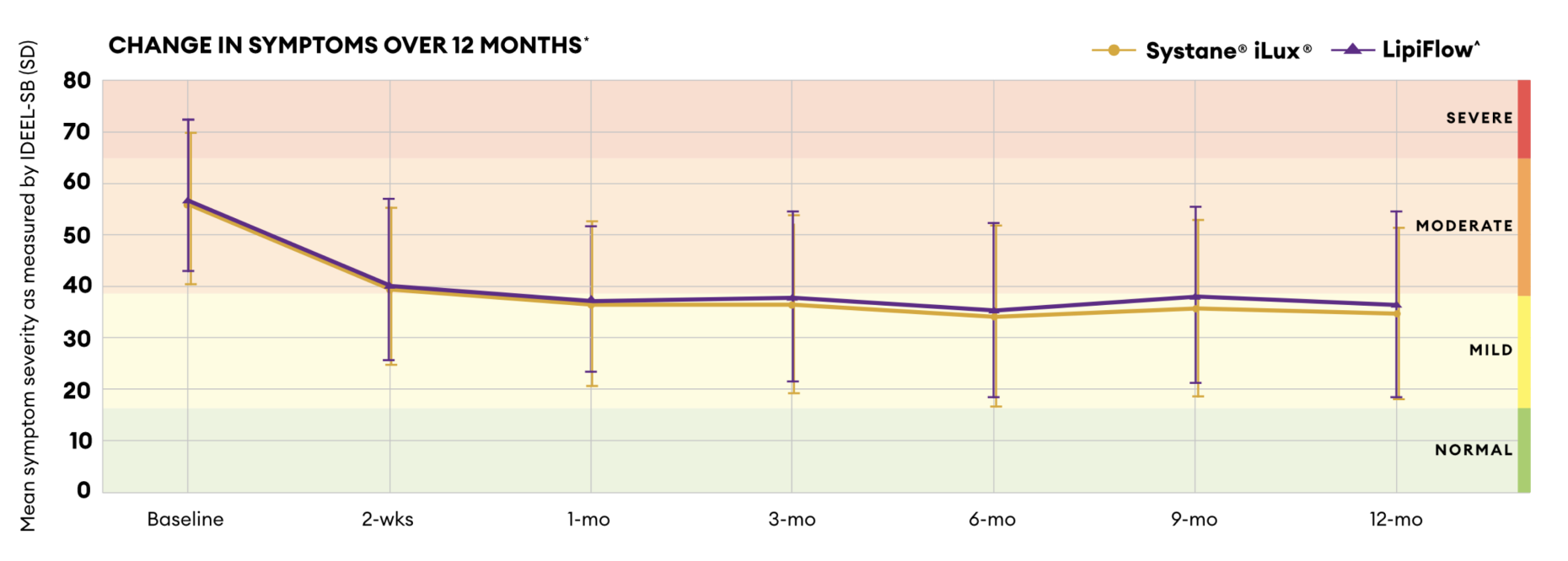
IDEEL-SB Score
Systane® iLux²® was shown to be non-inferior to LipiFlow^ over 12 months and maintained clinically significant improvements in signs and symptoms of MGD vs. baseline.3
Key Findings with Systane® iLux²®


Prospective, randomized, parallel group, investigator masked, multi-center, 12-month follow-up study. 236 subjects with meibomian gland dysfunction.3
*Impact of dry eye on every day life (IDEEL-SB) symptom bother score (with 20 questions).
^Trademarks are the property of their respective owners.
Meibomian Gland Score†
Systane® iLux²® was demonstrated to be non-inferior to Lipiflow^ for meibomian gland function through 12 months (at all measurements, including 1, 3, 6, 9 months).
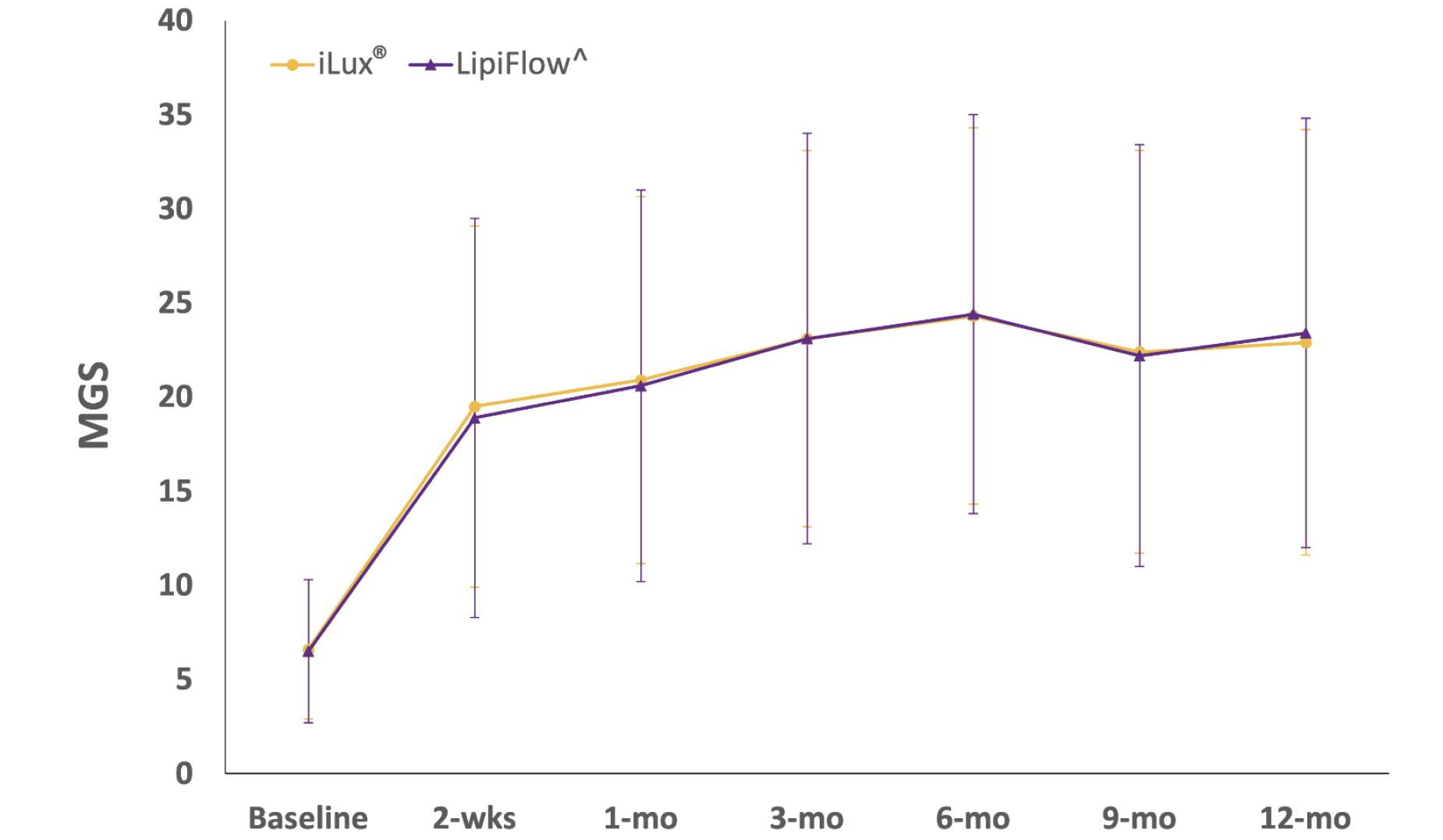
†Meibomian gland scores (0-45) measured using Meibomian Gland Evaluator to assess the 15 glands of the lower lid of each eye with a grade from 0-3.
††Impact of dry eye on every day life (IDEEL - SB) symptom bother score (with 20 questions).
^Trademarks are the property of their respective owners.
Summary
Non-inferiority of iLux® to LipiFlow^ was demonstrated in change from Baseline MGD scores(95% LCL of the LSM difference >-0.5), and change in Baseline IDEEL-SB module score (95% UCL of the LSM difference <12)
Prospective, randomized, parallel group, investigator-masked, multi-center 12-month follow-up study
Follow-up visits at 2 weeks and 1, 3, 6, 9, and 12 months

236 subjects with meibomian gland dysfunction
†Meibomian gland scores (0-45) measured using Meibomian Gland Evaluator to assess the 15 glands of the lower lid of each eye with a grade from 0-3.
††Impact of dry eye on every day life (IDEEL - SB) symptom bother score (with 20 questions).
^Trademarks are the property of their respective owners.
Meibomian Gland Procedures Are the Fastest Growing Part of the Dry Eye Market6


Approximately 44 million adults have dry eye symptoms.
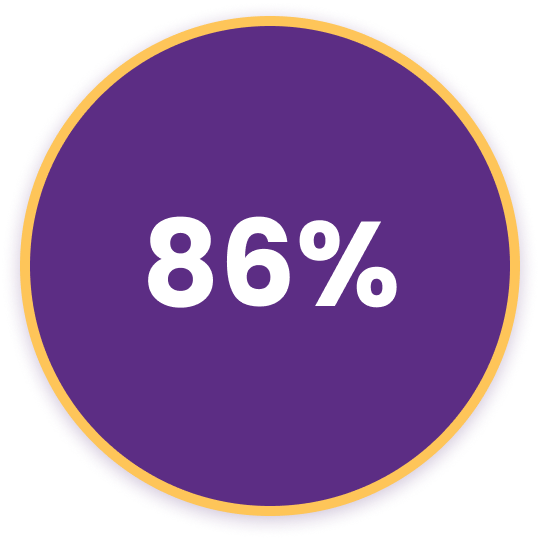

86% have signs of Meibomian Gland Dysfunction (MGD).7


Nearly 8 out of 10 doctors consider Dry Eye treatment as an opportunity to grow their practice.8*
*n=233 U.S. ODs, MDs.
Eye Care Practitioners Know They Need to Do More for Their MGD Patients—Systane® iLux²® Can Help You Do Just That.


Your patients
Use Systane® iLux²® meibomian gland imaging and HD video to help patients see and understand the need for in-office MGD treatment.


Your practice
An all-in-one, easy to use device with implementation support from Optometric consultants who can help you seamlessly integrate your device, leading to improved patient experience and retention.


Your bottom line
Introduce a new business model and a new treatment pathway that can add to your practice's bottom line.
Introduction Tutorial
Continue Your Patients' Dry Eye Care with Systane®, the #1 Doctor Recommended Brand of Artificial Tears.9
*vs. SYSTANE® BALANCE.

Systane® iLux2®
Important Product Information
The Systane® iLux2® is indicated for the application of localized heat and pressure therapy in adult patients with meibomian gland dysfunction (MGD), which is associated with evaporative dry eye, and to capture/store digital images and video of the meibomian glands.
Do NOT use the Systane® iLux2® in patients with the following conditions: Patients whose pupils have been pharmaceutically dilated; patients with ocular injury or trauma, chemical burns, or limbal stem cell deficiency (within prior 3 months); patients with active ocular herpes zoster or simplex of eye or eyelid or a history of these within prior 3 months; patients with cicatricial lid margin disease; patients with active ocular infection, active ocular inflammation or history of chronic, recurrent ocular inflammation within prior 3 months; patients with an ocular surface abnormality that may compromise corneal integrity; patients with lid surface abnormalities that affect lid function in either eye; patients with aphakia; or patients with permanent makeup or tattoos on their eyelids.
Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner.
The Disposable may not fit all eyes, such as eyes with small palpebral fornices. Use of the Systane® iLux2® is NOT recommended in patients with the following conditions: moderate to severe allergic, vernal or giant papillary conjunctivitis; severe eyelid inflammation; systemic disease conditions that cause dry eye; in patients who are taking medications known to cause dryness; patients with punctal plugs, or patients who have undergone ocular surgery within prior 3 months.
Potential adverse effects may occur because of the procedure. These effects include but are not limited to, the onset or increase in: eyelid/eye pain requiring discontinuation of the treatment procedure, eyelid irritation or inflammation, temporary reddening of the skin, ocular surface irritation or inflammation (e.g., corneal abrasion, conjunctival edema or conjunctival injection (hyperemia)), and ocular symptoms (e.g., burning, stinging, tearing, itching, discharge, redness, foreign body sensation, visual disturbance, sensitivity to light).
Attention: Please refer to the User Manual for a complete list of contraindications, instructions for use, warnings and precautions for the Systane® iLux2®.
References:
1. Tauber J, Owen J, Bloomenstein M, Hovanesian J, Bullimore MA. Comparison of the iLUX and the LipiFlow for the Treatment of Meibomian Gland Dysfunction and Symptoms: A Randomized Clinical Trial. Clin Ophthalmol. 2020;14:405-418.
2. Alcon data on file, 2019.
3. Wesley G, Bickle K, Downing J, et al. Comparison of Two Thermal Pulsation Systems in the Treatment of Meibomian Gland Dysfunction: A Randomized, Multicenter Study. Optom Vis Sci. 2022;99(4):323-332.
4. Geffen, D. iLux Efficacy after One Week of Treatment. Poster presented at American Academy of Optometry, Academy at Home, October 2020.
5. Hardten DR, Schanzlin JD, Dishler JG, et al. Comparison of a handheld infrared heating and compression device for treatment of meibomian gland dysfunction to a thermal pulsation device. Presented at the Annual Meeting of the American Society of Cataract and Refractive Surgery (ASCRS); April 13–17, 2018; Washington, D.C.
6. MarketScope LLC. 2020 Dry Eye Products Report: A Global Market Analysis for 2019 to 2025. St. Louis, MO: MarketScope LLC; 2020.
7. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472-478.
8. Alcon data on file, 2018.
9. Based on IQVIA ProVoice Survey of General Practitioners, Eye Care Professionals and Pharmacists, 12 months ending December 31, 2021.


